Abstract
Introduction. Since publication of the pivotal CLL011 phase 3 trial, combination of chlorambucil and anti-CD20 MAb has become the standard first-line CLL treatment for patients unfit for FCR. Lenalidomide (Len) has been proven effective both as monotherapy and in combination with rituximab. Although recent studies in CLL suggest that Len is mostly beneficial as consolidation treatment, the exact place and duration of this drug is still not optimized. Moreover, Len has shown a distinct and more difficult to manage toxicity profile in the context of CLL, potentially hampering combination treatment with this drug. We therefore conducted a phase 2 study to evaluate efficacy and safety of a combination of chlorambucil, rituximab and individual dosed Len (induction-I) followed by 6 months of Len monotherapy (induction-II).
Methods. Treatment-naïve patients were eligible for this study if they had treatment indications per IWCLL 2008 criteria, WHO PS ≤2 and adequate hepatic and renal function. Initially, a phase 1 study was performed to determine the maximum tolerated dose of chlorambucil in combination with rituximab and Len. Although no major toxicities were observed with chlorambucil at a dose of 10mg/m2 on days 1-7 in 6 patients, a trend was observed of decreased tolerability of Len. Therefore, the phase 2 part of the study was conducted with chlorambucil at a dose of 7mg/m2 on days 1-7, cycle 1-6. Rituximab was given IV at day 1 at a dose of 375mg/m2 cycle 1 and 500mg/m2 cycle 2-6. Len was started on day 9 of cycle 1 at 2.5 mg orally and from then on administered daily and continuously. At cycle 2, Len was increased to 5 mg and at cycle 3 to a maximum of 10 mg or continued at the maximum tolerated dose. Following 6 cycles of combination treatment, patients continued Len monotherapy for 6 cycles of 28 days. A CT-scan was performed after 6 and 12 cycles, MRD was measured on peripheral blood after cycle 12. Antithrombotic prophylaxis (acetyl salicylic acid) was mandatory and support with g-CSF was to be given in case of grade ≥3 neutropenia. The primary endpoint was ORR.
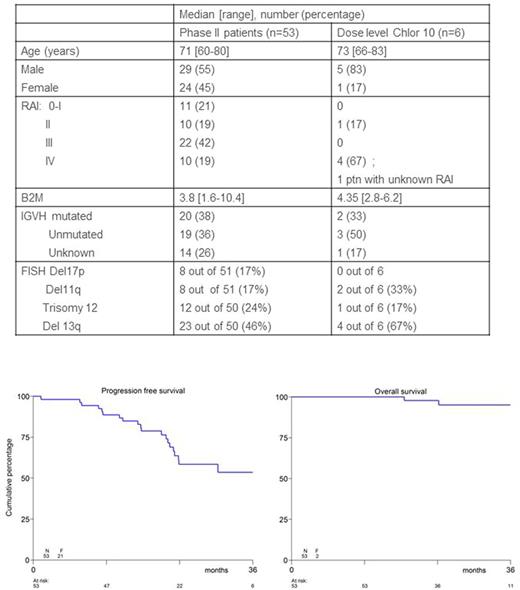
Results. 63 patients were enrolled of whom 57 in the phase 2 part of the study. As 4 patients were not eligible (SLL, not CLL), all further analyses were performed on 53 patients. Baseline patients characteristics are shown in the Table.
47 patients (89%) completed induction-I and 36 patients (68%) completed induction-II. Reasons for dropout were progression (n=1), excessive toxicity (n=8) and refusal (n=2) during or after induction-I and excessive toxicity (n=5) and refusal (n=1) during induction-II. During Induction-I, full dose of Len dosed to 10mg could be given to 56% of patients in cycle 3, ,64% in cycle 4, and 57% in cycle 6. At cycle 12 of Induction-II 75% of remaining 36 patients received Len at the full dose.
ORR after induction-I was 83% (all PR) and for the 42 patients who started induction II, the ORR after induction-II was 93% (14% CR). Depth of response improved after induction-II in 8 patients (2 from SD to PR and 6 from PR to CR). Flow-based MRD could be performed in 41 patients, 4 achieved MRD negativity.
The 3-year PFS was 54% and the 3-year OS was 95% with a median follow-up of 27 months (see figure).
Tumor lysis syndrome (TLS) and tumor flare reaction (TFR) have been of specific concern in earlier CLL trials incorporating Len. Using the individualized dose schedule, no TLS was observed, while TFR occurred in 5 patients (9%, one grade 3). As for hematologic toxicities, grade 3-4 neutropenia, thrombocytopenia and anemia occurred in 36%, 6% and 0% of treatment cycles respectively at induction-I and 33%, 6% and 0% at induction-II. Other grade 3-4 toxicities occurred in 44% of patients during induction-I (top 3: general, skin, and gastrointestinal (GI)). At induction-II 19% of patients experienced grade 3-4 toxicities, of which 10% infections and 7% GI.
Another Len-specific concern has been second primary malignancies (SPM). In our study cohort 6 patients with SPM were observed (all but one a localized skin cancer).
Conclusions. Addition of Len to a backbone of chlorambucil and rituximab followed by a fixed duration of Len monotherapy in 1st line FCR-unfit patients results in high remission rates with PFS rates that seem comparable to those observed with novel combinations including novel CD20 MAb or kinase inhibitors. Although Len-specific toxicity remains a concern, an individualized dose-escalation schedule is feasible and results in an acceptable toxicity profile.
Kater: Celgene: Consultancy, Research Funding; Johnson & Johnson: Research Funding; Abbvie: Research Funding. Van Oers: Roche: Consultancy; ISA therapeutics: Consultancy; Novartis: Consultancy; Immunicum: Consultancy. Schipperus: Amgen: Research Funding. Chamuleau: Genmab: Research Funding; Gilead: Research Funding. Nijland: Novartis Pharmaeuticals Corporation: Honoraria; Kite Pharma: Honoraria; Roche: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; MIllennium/Takeda: Honoraria, Research Funding; Mundipharma: Honoraria; Gilead Sciences: Honoraria; BMS: Honoraria; MSD: Honoraria; Amgen: Honoraria. Doorduijn: Roche: Honoraria; Celgene: Honoraria. van Gelder: Roche: Honoraria. Raymakers: Roche: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal